The changing technologies of drug design Understand article
Over several decades, the search for new medicines has progressed from mimicking natural molecules to screening many millions of compounds.
The first medicines chemists designed were inspired by nature. Aspirin was derived from an extract of willow; belladonna came from deadly nightshade; opioid medicines such as morphine have their roots in the opium poppy. Later, medicines were found in other natural materials – notably penicillin, which was isolated from a mould.
While nature has continued to be a rich source of ideas for new drugs, there are many diseases for which no natural compounds are available as treatments. Scientists now have a key role in designing new compounds that can lead to effective pharmaceutical treatments. This has required the adoption of new approaches and techniques – from automated screening to genetic engineering – to develop a drug discovery process that is more complex and precise than ever before.
Designing modern medicines
Understanding how biochemical processes work is central to modern medicine design. By studying the biochemistry of a particular effect, scientists can design molecules that either mimic the effect or stop it from happening. Since most of the processes in our bodies involve proteins, scientists want to create drugs that alter the behaviour of the proteins associated with a particular disease.
The first new medicine to be invented in this way, in the early 1960s, was the beta-blocker propranolol. When a patient takes propranolol, the drug sits in the beta receptors of cells and stops adrenaline from binding. Because it blocks the natural activity of adrenaline, propranolol has a range of uses – from lowering blood pressure to preventing migraines.
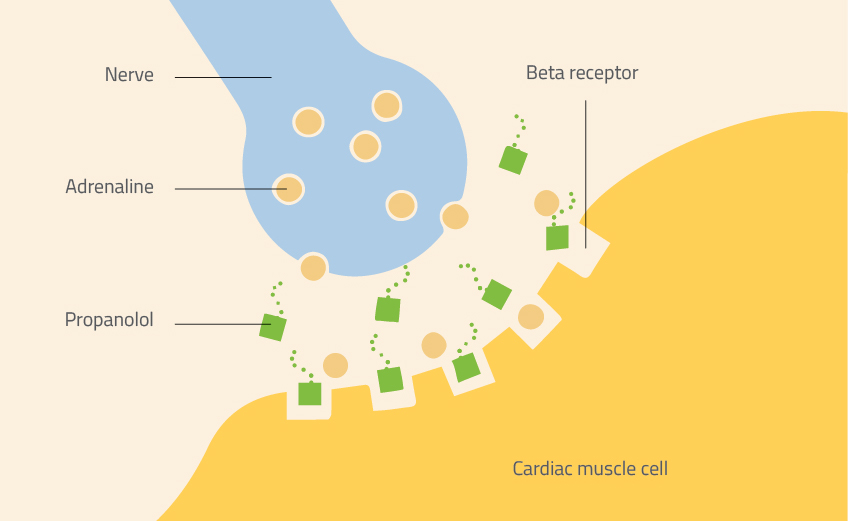
Nicola Graf
By the late 1980s, scientists could isolate individual proteins using new molecular biology techniques. This allowed them to study and measure the strength and nature of the binding interactions between proteins and potential drug molecules. Techniques included adding fluorescent tags or radiolabels to the protein or the drug molecule, enabling them to be tracked. The inspiration was the chemical structure of the natural hormone that fits into the target receptor: its shape, and its functional groups, provide clues about the shape that a drug molecule must have in order to fit into the receptor. Chemists could also design modifications to that natural structure that might alter its activity as required.
At first, this process was very much a case of trial and error. But over time, much knowledge has been built up about how different functional groups can affect activity at receptors. Today, chemists can predict what changes they need to make to a molecule’s structure to achieve the desired response. By accurately modifying the design of natural molecules, chemists can improve their affinity (how well they bind to the receptor), activity (how well they work) and selectivity (so they do not act at different receptors as well). The chemists will also do their best to tweak molecules to make them less likely to cause side-effects.
Molecular starting points
Nowadays, however, chemists don’t always use the natural hormone as their molecular starting point. Instead, they test the target receptor against huge ‘libraries’ of different compounds. In an automated process called high-throughput screening, tests are carried out to find molecules that have the desired activity in the receptor. In this very rapid process, screening a million compounds in a week is not unusual. The results of these tests then provide chemists with an all-important starting point for designing drugs.
High-throughput screening finds molecules that interact reasonably strongly with the protein target, but they are often fairly large. To identify small chemical fragments – which typically produce a weaker effect – scientists instead use a similar process called fragment-based drug discovery. Rather than looking for molecules that already resemble drugs, like the molecules in a normal compound library, the idea is to use smaller, simpler molecules that can be developed into more complex molecules later on. This approach can give a better insight into which functional groups have a biological effect, because a small molecule has fewer parts to interact with the target. Also, attributes from more than one fragment can ultimately be combined into a single complex molecule.
Two anticancer drugs derived from fragment-based drug discovery have made it to market – vemurafenib and venetoclax. Several more are undergoing clinical trials.

National Center for Advancing Translational Sciences/Flickr, CC BY 2.0

Daniel Soñé Photography, LLC/National Center for Advancing Translational
Sciences/Flickr, CC BY 2.0
Phenotypic screening
In recent years, there has been something of a return to the old approach to drug discovery: identifying a physiological effect and then working out which biological process caused it. Instead of an isolated protein, chemists use a cell, a tissue, or even a whole organism. This is known as phenotypic screening, because it identifies the molecules that alter the phenotype of that cell, tissue or organism.
For example, in cancer, the physiological effect is cells multiplying, and the phenotype to change is cell growth, so a suitable drug molecule will kill cancer cells or arrest their growth. Or in diabetes, researchers might take a pancreatic islet cell and look for molecules that alter glucose-stimulated insulin secretion.
Once the molecules have been identified, the next step is to work out what happens when they are added to the cell, tissue or organism. Advances in chemical biology now allow us to look inside the cell and see how the molecules bind to proteins within it. Today, multiple molecular processes can be observed and studied at the same time, whether they are taking place within cells or on their surface.
Extremely sensitive mass spectrometry and fluorescence methods are the key to these advances, as they help to detect individual proteins among the thousands expressed by a cell.
Modern genetic techniques
Advancements in genetic engineering and gene editing have also helped to progress the discovery of new drugs. Using modern DNA editing techniques, such as TALENs and CRISPR, scientists are able to remove, insert or replace genes in a very precise way. Scientists can use these very targeted genetic modifications to edit the genome of cells, giving them confidence that what they see as a result of phenotypic screening is the effect they want to see and not something else.
Gene editing can also be used to modify a binding site and see if it stops the original biological process from happening. Combinations of these new techniques have sped up research that, in the past, would have taken many years, if not decades. Genetic engineering thus has the potential to transform the way scientists search for drugs in the future.

Orawan Pattarawimonchai/Shutterstock.com
Acknowledgement
This is an edited version of an article first published in the magazine Education in Chemistry in May 2017w1.
Web References
- w1 – Education in Chemistry is a magazine for chemistry teachers, published by the Royal Society of Chemistry in the UK. To see the original article, visit the EiC website.
Resources
- Learn about the steps in the drug development process from molecule screening to marketing in an article from Tomorrow’s Pharmacist.
- Watch a TED-Ed video about the discovery of aspirin.
- Explore the trial-and-error process of chemical modification in drug design with a printable drug discovery game.
Review
The design of new drugs is an exciting subject of chemistry in the 21st century. Research in this field involves designing specific molecules that act locally at the molecular level.
This article can be used to introduce current methods of searching for and designing new drugs, particularly to advanced students. Teachers can use the article to discuss the methods from different fields of science that are now being used to modify the mechanisms of human body function.
Through these methods, the article makes links between chemistry, biology and even philosophy. It could also be used to prepare secondary school science students for future university courses, or as a complementary lecture for the final courses.
Comprehension questions teachers could use include:
- Today, where do the first ideas come from in designing new drug molecules?
- Why is combining different techniques a good way to design new drug molecules?
- Side-effects are not desirable when we take drug treatments. How can chemists help to avoid side-effects in new drugs?
Manuel Hernández Rodríguez, chemistry teacher, IES Pedro Mercedes school, Cuenca, Spain
