Fake news in chemistry and how to deal with it Understand article
What's wrong with 'chemical-free' labels? Is ‘natural’ necessarily better? Learn how to spot pseudoscientific fake news in the media.
Fake news involves the spread of misinformation, typically with the aim of creating confusion in people’s minds, or discrediting a person or an institution. Fake news is a major challenge in society these days due to the rapid spread of information through the internet and social networks. Fake news is a particular problem in topics related to health, where those spreading fake news often use pseudoscience to confuse people. This is where explanations are presented as ‘science’ even though they have no basis in scientific evidence, for example, the so-called alkaline diet, which falsely claims that ‘alkalizing’ foods and ‘ionized water’ can cure cancer.[1] Let’s look at some further examples of fake news and misinformation relating to chemistry.[2]
Fake news in food chemistry
Food is essential for life and most people know a little about very basic food chemistry, for example, they have heard of the main macronutrients: carbohydrates, fats, and proteins. However, people generally have far less understanding of the other chemical compounds present in food and their role, which makes it easy for them to be fooled by pseudoscientific marketing claims.[3] A very popular type of food fake news is an exaggeration of the health properties of a specific food or product to induce people to buy more of it.[4] An example of this is ‘pink salt’, also called Himalayan salt, which is extracted from a mineral cave in Khewra in Pakistan (actually quite far from the Himalayan mountains).
The case of pink salt: exaggerated health properties for marketing
Marketers promoting pink salt over common white salt claim that it has positive health effects. In fact, pink salt is 98–99% sodium chloride (NaCl), the same chemical compound as white salt. The rest (less than 2%) is a mix of minor mineral elements, such as cadmium (Cd), copper (Cu), nickel (Ni), lead (Pb), and iron (Fe).[5] The presence of small amounts of iron oxides (commonly known as rust) is the origin of the pink colour. Pink salt is, ultimately, very similar to white salt, with the addition of some impurities that do not add special health properties. In fact, impurities of toxic lead and cadmium could actually make pink salt less healthy. Furthermore, high salt consumption is unhealthy, and salt can even be deadly in high concentrations. [6,15]
 | Trace chemical elements found in pink salt: Iron (Fe), Zinc (Zn), Copper (Cu), Manganese (Mn), Chromium (Cr), Lead (Pb), Cadmium (Cd), Nickel (Ni), Cobalt (Co), Silver (Ag), Aluminium (Al), Boron (B), Barium (Ba), Bismuth (Bi), Gallium (Ga), Indium (In), Lithium (Li), Strontium (Sr), Tellurium (Te). |
Image: pictavio/pixabay.com
Natural is good, chemical is bad?
A widespread misconception at the heart of lots of fake news in chemistry is the idea that ‘chemistry’ and ‘chemicals’ are associated with synthetic or artificial substances, in contrast with ‘natural’ ingredients or products. Scientifically speaking, all substances, whether synthetic or natural, are made of chemicals.[8] The misconception that sets chemical against natural is related to a distorted negative image of chemistry known as chemophobia.[3, 9,10] This idea is also behind the false belief that synthetic chemicals are more harmful than natural ones.[11]
In people’s minds, the word natural is associated with things that are safe and healthy, whereas the terms chemical, synthetic, and artificial are associated with negative or harmful properties, and marketing teams know this well! This is the reason many products have ‘chemical-free’ labels. However, no foods or substances are chemical-free; this label is fake news!
If you look at the ingredients list of a food product (e.g., biscuits), you can probably find terms like natural flavours, aroma, or fragrances: these words are used instead of more technical names, which people instinctively associate with ‘nasty chemicals’. In other cases, chemical names and formulae are replaced with more neutral labels, such as E numbers.[12] These labels were introduced in Europe for all food additives that at some point were approved by the European Food Safety Authority (EFSA). [13] For example, packaged meat usually contains the additives E301 and E331, which are classified as antioxidants.[12] Due to chemophobia, the chemical names of these two additives (sodium ascorbate and sodium citrate) can sound scary to uninformed consumers. It was hoped that the simpler E-numbering system would help, but in fact the term ‘E numbers’ is now commonly used as a shorthand for harmful additives. This is nonsense since an E number tells you nothing about the origin of the chemical compound; compounds with E numbers can be natural or artificial. Sodium ascorbate and sodium citrate can be found in nature or produced in a laboratory and their properties are identical.
It should be noted that although some compounds with E numbers are harmless, some are controversial or have been withdrawn as food additives in Europe (and other countries) based on updated evidence. The E-number system is nothing more than a naming system; it tells you nothing about whether or not a substance is harmful.
| ‘E’ label | Chemical name | Properties | Status |
|---|---|---|---|
| E101 | Riboflavin (Vitamin B2) | Food colouring (yellow); found naturally in eggs and other foods but also produced synthetically | Approved in Europe and USA. |
| E160a | β-Carotene | Food colouring (yellow-orange); naturally present in many fruits and vegetables | Approved in Europe |
| E230 | Biphenyl / diphenyl | A food preservative | Not approved in Europe |
| E250 | Sodium nitrite | A salt; used as a food preservative | Approved in Europe |
| E300 | Ascorbic Acid (Vitamin C) | An antioxidant; found naturally in fruits and also produced synthetically | Approved in Europe |
| E354 | Calcium tartrate | A byproduct of the wine industry; used as emulsifier | Approved in Europe |
Toxic? It’s a matter of dose
The general public often misunderstands what is meant by toxicity.[14] The potential danger of chemicals, either natural or synthetic, is very much related to their concentration. This was known even in historical times: in 1538, the alchemist Paracelsus wrote, “All things are poison, and nothing is without poison, the dosage alone makes it so a thing is not a poison” (translated from German).[14] Substances that are widely considered safe, such as caffeine, or even beneficial substance like vitamins, can be toxic if they are consumed in large amounts. Even water can be lethal when a large amount is drunk in a short time (or when it is inhaled!). The quantity of the substances is key to determining their effect on human health.
Moreover, the fact that a substance is present in nature doesn’t mean that it is safe. A large number of natural toxins are known that are lethal even in very low concentrations. [15] For example, many fungi, plants, and bacteria produce compounds that act as natural pesticides, and some are also highly toxic to humans.
| Cycloprop-2-ene carboxylic acid Found in mushrooms, such as Russula subnigricans. It is highly toxic for humans and causes rapid muscle breakdown; just a few bites of this mushroom can be fatal. | 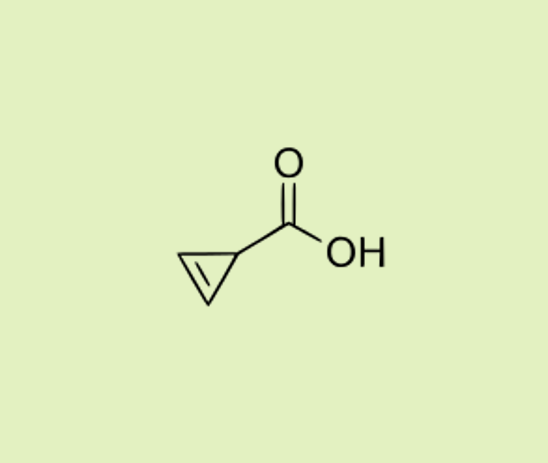 |  |
| Aflatoxin B1 Produced by the fungus Aspergillus flavus, which can grow on foods like peanuts and grains. It is highly carcinogenic (cancer-causing). | 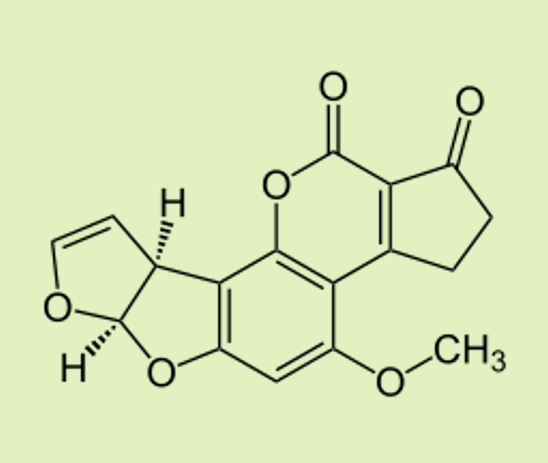 |  |
| Coniine A highly toxic alkaloid compound found in the poison hemlock plant (Conium maculatum), which famously killed the philosopher Socrates. | 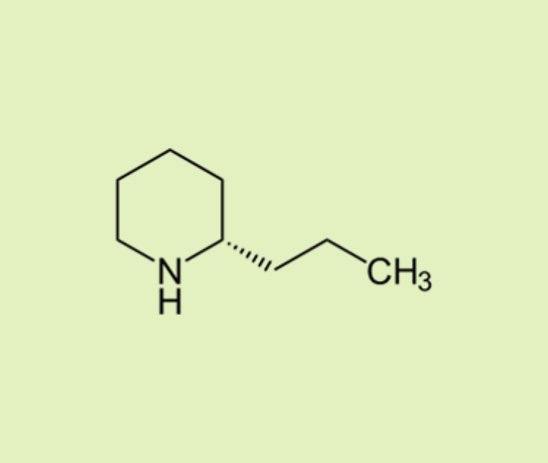 |  |
| Nicotine Produced by tobacco plants (Nicotiana species). High doses can also cause dangerous nicotine poisoning. It was previously used as a natural pesticide but this is now banned in the EU and many other countries. | 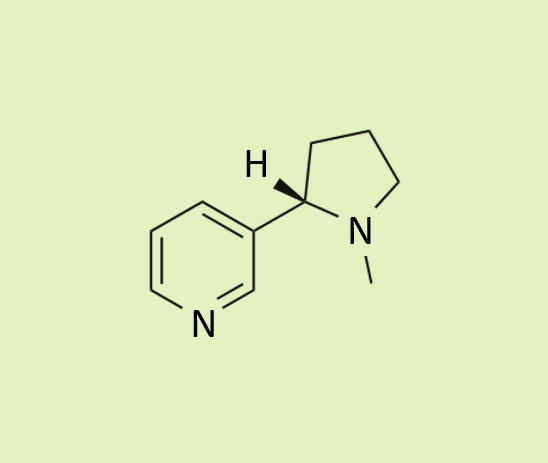 |  |
Images: Russula subnigricans: pumakit/iNaturalist, CC BY 4.0: Aspergillus flavus on maize: International Institute of Tropical Agriculture/Flickr, CC BY-SA 2.0. Poison hemlock: Djtanng/Wikipedia, CC BY-SA 4.0. Tobacco plants: Kotoviski/Wikipedia, CC BY 3.0.
How can we recognize fake news in chemistry?
The consequences of misinformation when it comes to health topics can be very serious. For example, cancer patients may try ineffective alternative therapies rather than accepting life-saving medical treatment,[18] or people might refuse vaccination, leading to serious public health consequences.[19] The amount of information online can be confusing but there are some strategies that can be used to recognize fake science news. Fact-checking is the first step. Remember that anyone can share anything on the internet! If you are not familiar with the topic, a good idea is to see whether the information can be verified from reputable sources like government health agencies such as the Centers for Disease Control and Prevention (CDC) or European Food Safety Authority (EFSA) ; scientific bodies such as the Royal Society of Chemistry (RSC); or universities[20] or research institutes.[21] A general approach to confronting fake news was proposed by Mike Caufield, an expert in network learning, who coined the term SIFT: Stop, Investigate the source, Find additional coverage, and Trace claims back to the original source.[22] Here are some practical tips:
- STOP. Pause before reading to consider whether the sources are reliable. Do not believe a media story if references and sources are not given.
- INVESTIGATE THE SOURCE. Check who is posting the information (e.g., in the website ‘about’ section). Ask whether the author might have something to gain, for example, are they part of or sponsored by a company selling a product related to the content? Influencers and other content creators that are paid according to their follower numbers can also benefit from the audience that ‘clickbait’ articles bring.
- FIND ADDITIONAL COVERAGE. Follow any provided references and links and check that they are genuine and actually support the claims made. Try to find different sources for the same story and see if the interpretations differ.
- TRACE CLAIMS TO THE ORIGINAL SOURCE. Sometimes references are out of date or contain disproven claims. If the references are scientific papers and you are not able to access them, look up the authors and their institutes. Also check that the paper is in a reputable peer-reviewed journal.
These steps cannot tell you with 100% accuracy whether an article or post contains fake news, but your chances of being fooled will be considerably reduced.
References
[1] An article on Science-Based Medicine debunking the claims on health effects of alkaline water: https://sciencebasedmedicine.org/alkaline-water-surges-despite-lack-of-evidence/
[2] An article on Chemistry World about tackling fake scientific news: https://www.chemistryworld.com/opinion/how-can-we-tackle-fake-science-news/4010598.article
[3] Siegrist M Bearth A (2019) Chemophobia in Europe and reasons for biased risk perceptions. Nature Chemistry 11 1071–1072. doi: 10.1038/s41557-019-0377-8
[4] The marketing hype surrounding ‘superfoods’: https://www.hsph.harvard.edu/nutritionsource/superfoods/
[5] Sharif QM, Hussain M, Hussain MT (2007). Chemical Evaluation of Major Salt Deposits of Pakistan. Journal-Chemical Society of Pakistan 29: 569–574.
[6] Martin TP, Fischer A. (2012) SODIUM, POTASSIUM, AND HIGH BLOOD PRESSURE. ACSM’s Health & Fitness Journal 16: 13–21. doi: 10.1249/01.FIT.0000414751.69007.b5
[7] An answer on WebMD to the health claims made on Himalayan salt: https://www.webmd.com/diet/himalayan-salt-good-for-you
[8] A post listing all the chemicals that are found in a banana: https://jameskennedymonash.wordpress.com/2013/12/12/ingredients-of-an-all-natural-banana/
[9] How to recognize (and talk to) a chemophobe: https://blogs.scientificamerican.com/the-curious-wavefunction/how-to-recognize-and-talk-to-a-chemophobe/
[10] Saleh R, Bearth A, Siegrist M (2020) Addressing chemophobia: informational versus affect-based approaches. Food and Chemical Toxicology 140: 111390. doi: 10.1016/j.fct.2020.111390
[11] Shim S-M et al. (2011) Consumers’ knowledge and safety perceptions of food additives: Evaluation on the effectiveness of transmitting information on preservatives. Food Control. 22: 1054–1060. doi: 10.1016/j.foodcont.2011.01.001
[12] The Wikipedia page on food additive codes used within the European Union and the European Free Trade Association: https://en.wikipedia.org/wiki/E_number
[13] The European Food Safety Authority (EFSA)’s website https://www.efsa.europa.eu/en
[14] An article about toxicity and the dose-response relationship: www.sciencelearn.org.nz/resources/365-all-in-the-dose
[15] Gribble GW (2013) Food Chemistry and Chemophobia. Food Security 5: 177–187. doi: 10.1007/s12571-013-0251-2
[16] Information on coniine: https://natoxaq.ku.dk/toxin-of-the-week/coniine/
[17] The dangers of nicotine in agriculture and food: https://www.ua-bw.de/pub/beitrag_printversion.asp?subid=0&Thema_ID=5&ID=2963&Pdf=No&lang=EN
[18] An article on the dangers of alternative cancer therapies from the National Cancer Institute: https://www.cancer.gov/news-events/cancer-currents-blog/2017/alternative-medicine-cancer-survival
[19] The problem of vaccine hesitancy: https://www.criver.com/eureka/vaccine-hesitancy-story-old-vaccines-themselves
[20] The McGill University Office for Science and Society has really nice articles explaining the science behind everyday issues: https://www.mcgill.ca/oss/our-articles
[21] The Mayo Clinic offers a lot of curated information on health-related topics: https://www.mayoclinic.org/
[22] An article in the American Chemical Society magazine ChemMatters on reading science news and spotting misinformation: https://www.acs.org/content/acs/en/education/resources/highschool/chemmatters/past-issues/2021-2022/october-2021/reading-science-news.html
Resources
- Read two excellent articles on spotting misinformation in science news and finding trustworthy health information.
- Learn about the importance of peer-reviewed science from Sense about Science.
- Explore the resources provided by the Ask for Evidence campaign, such as this guide on making sense of chemical stories.
- Try this evidence hunter activity for high school students.
- Discover all the chemicals that are naturally found in a banana. It might surprise you!
- Watch a food scientist’s reaction to viral videos with fake statements about food.
- Watch a video on the claims that salt lamps are good for you.
- Watch a video on the toxicity of vital substances (like water and oxygen) when the quantity is too high.
- Read about the dihydrogen monoxide hoax, illustrating how easy it is to spread fear even when talking about water.
- Read an analysis of the response of the blogger ‘The Food Babe’ to criticisms of her unfounded science claims by David H. Gorski, oncologist and professor of Surgery.
- Read an excellent article by the McGill Office for Science and Society (OSS) on some of The Food Babe’s nonsensical claims about natural flavours.
- Watch an America Chemical Society webinar about dealing with chemophobia (more for teachers than students).
- Read an RSC article on how teachers can address chemophobia.
- Find inspiration in a group of students that stood up to The Food Babe and asked for evidence.
- Read about the colour pink in nature and the chemistry behind it: Bettucci O (2022) Colour in nature: think pink. Science in School: 56.
- Read about the importance of statistics and correct data analysis: Le Guillou I (2021) Clinical trials count on more than statistics. Science in School: 52.
- Investigate the properties of the so-called superfoods: Frerichs N, Ahmad S (2020) Are ‘superfoods’ really so super?Science in School 49: 38–42.
- Explore the biochemistry of bananas: Glardon S, Scheuber T (2018) Go bananas for biochemistry. Science in School44: 28–33.
- Investigate food chemistry with mushrooms: Bunjes F et al. (2017) Natural experiments: chemistry with mushrooms. Science in School42: 36–41.
- Discover the science of limonene: Butturini F, Fernández JJ (2022). Citrus science: learn with limonene. Science in School: 58.
- Learn how to explore chemistry with tea: Prolongo M, Pinto G (2021) Tea-time chemistry. Science in School: 52.
Review
The following article provides a review of fake news pertaining to chemistry in society. From fake marketing myths of chemical-free food to the idea that everything is toxic in certain amounts, the article provides a good introduction to the misconceptions that day-to-day consumers face.
For students it might be a first point of reflection on what “pure”, “toxic” and “chemical-free” actually mean inside a science classroom.
This could be a really good introduction to a research project to use for homework. Students could use this as a prompt to research a specific instance where chemical claims are used in advertising and then follow steps at the end to determine whether it was fake news or not. Within the age of COVID-19 and TikTok where fake news is present at every corner, empower your students to be science myth busters in your community.
Suggested comprehension questions:
1) What additional minerals are found in pink Himalayan salt?
2) What are the potential risks of consuming too much pink salt?
3) Suggest four steps that can be taken to spot and tackle fake news?
4) Give two examples of natural toxins?
5) Define chemophobia
Valentina Valenza, Chemistry Teacher, UK


