The dark side of fireworks Understand article
Fireworks release more than just sound and light. Read about the environmental costs of this centuries-old entertainment.
Glittering greens, sparkling silvers, rocketing reds – nothing screams celebration like fireworks. All over the world, these colourful explosions are a regular feature of national holidays, religious festivals, and the start of a new year.

Thanks to improved technology, firework displays have become increasingly spectacular. However, for all of that, the basic chemistry behind fireworks has remained remarkably similar for a long time.
Fireworks were first invented in China about a thousand years ago, following the discovery of gunpowder. When the gunpowder was placed in a tube and lit with a fuse, it would create a loud explosion that was used to celebrate family occasions.
Over the centuries, we learned to fire the explosives into the air and produce a variety of colours. The spectrum of colours that we see in fireworks comes from metal compounds as they burn in the air. For instance, strontium gives us red while copper provides blue-green.
Some of the energy from the combustion reaction is transferred to electrons in the metal ions, which are excited to a higher energy state. These excited electrons then return to their ground state, releasing the energy as a photon of light. The wavelength or colour of the light depends on the gap between the electron energy states, which is different for each metal.
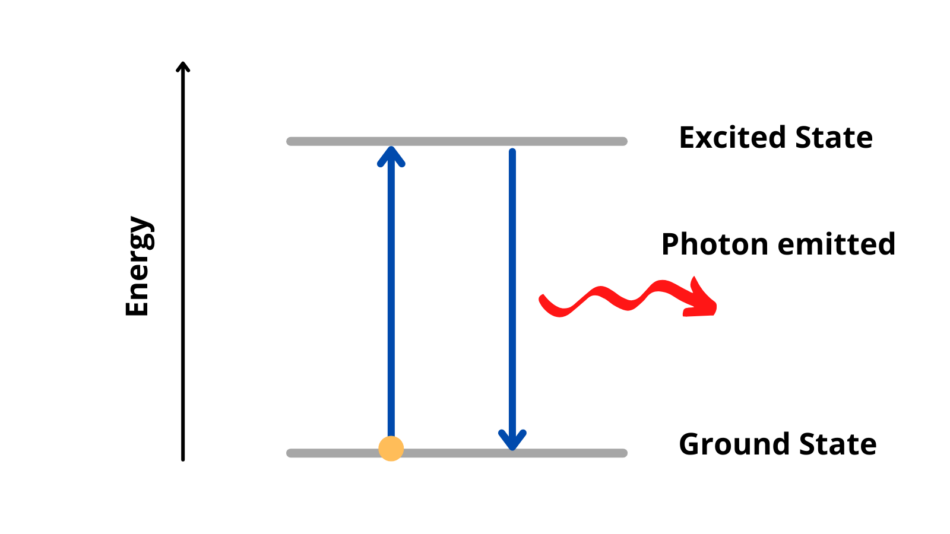
This is the same process that allowed scientists in the 1800s, like Robert Bunsen of Bunsen burner fame, to distinguish between different metal compounds in flame tests.

Image: Hegelrast/Wikimedia, CC BY-SA 4.0
Fireworks are affordable like never before, and many countries even allow personal use by ordinary citizens. As firework displays have grown bigger and more widespread, scientists have tried to understand the environmental impact of launching these burning chemicals into the skies above us.
Metals
Potassium is released in higher amounts than any other metal because it is used in compounds known as oxidants to make the fireworks burn, rather than provide colour. Estimates in the United Kingdom suggest that fireworks accounted for 28% of potassium emissions in 2019. [1] However, potassium is not considered to be hazardous and is an important natural mineral for plants.
Some other metals released, such as copper, are already common pollutants in urban environments. In these cases, fireworks do not make much difference to the total levels of pollutants. However, looking at the total amount of metal released does not give us the full picture. Fireworks don’t tend to be spread out across the year and are released in huge numbers over short periods for national and international celebrations. This means we need to consider the short-term acute impact as well.
A study in Spain of the Sant Juan festival found that the metals released by the fireworks can linger in the air around the area for days afterwards. [2] In particular, levels of strontium were 86 times higher than normal. Strontium is rarely produced by other polluting sources, so this can act as a good environmental marker for firework activity, especially as those red colours are so popular.
There is still limited information about whether these levels are harmful to us if we breathe them in. Long-term health effects can be influenced by so many different genetic, lifestyle, and environmental factors that it would be difficult to assign blame to firework pollution. However, scientists can analyze the pollutants for signs that they could do harm.
One study in London during Guy Fawkes night and Diwali, both celebrations that feature a lot of fireworks, found that particles in the air containing metals from the fireworks were more reactive than particles from traffic pollution.[3] This meant they could react more strongly with antioxidants found in the lungs, which protect our cells from damage. While there wasn’t evidence for lung damage, this indicates a potential mechanism that should be studied further.
Taking a breath
Beyond the metals used to produce the colours, burning fireworks also releases gases and fine particles. A study in Spain during the Las Fallas celebrations found a short-lived increase in pollutants, including small particles, nitrogen oxides, and sulphur dioxide.[4] These pollutants can all worsen asthma symptoms and are found at high levels in many cities, such as Milan and Warsaw, primarily from traffic and energy-generation emissions.[5]

Image: Mike Enerio/Unsplash.com
Even staying indoors during a firework festival only provides partial protection against some of these pollutants. Scientists in the USA measured levels of small particles known as PM2.5, which are 2.5 micrometres across or smaller, during the 4 July Independence Day fireworks. These fine particles can get deep into your lungs and even into the bloodstream, increasing the risk of heart and lung disorders. In an air-conditioned office, where the air had passed through filters, PM2.5 particles reached levels that are unhealthy for sensitive groups, such as children and older adults.[6] However, this was much lower than the readings outdoors, which hit levels regarded as very unhealthy for the general population.
Fire and water
One common ingredient in fireworks are perchlorates, which are highly reactive chemicals containing chlorine and oxygen. These are used to propel the fireworks into the sky and are also a component of rocket fuel. However, in our bodies, perchlorate can block iodide ions from reaching the thyroid gland, which is important for hormone production.
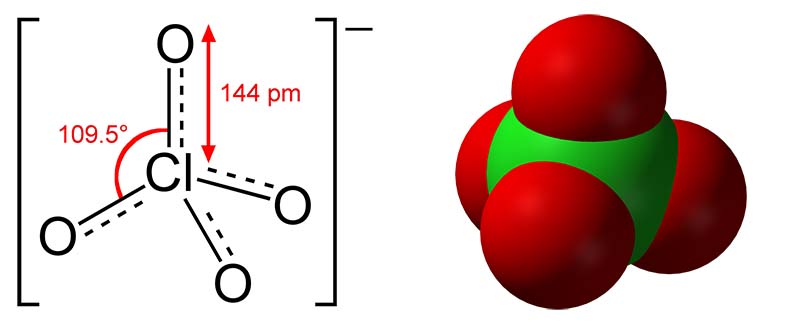
Scientists in the USA examined perchlorate levels in the water next to a firework display site in 2006.[7] They found that the concentration of perchlorates increased by up to 1000 times the usual levels within 14 hours after the fireworks. The highest concentration was 44 micrograms per litre, seen after the 4 July celebrations; this is well above the 18 micrograms per litre measure that the US Environmental Protection Agency describes as a potential level of public concern in drinking water. This is a particular problem since firework displays are often held next to bodies of water to minimize the risk of fire.
Wildfires are also often caused by fireworks, particularly in hot, dry areas in the summer. Researchers analyzing wildfires in the western United States between 1992 and 2015 discovered a sharp spike on 4 July.[8] Almost three times as many human-caused wildfires happen over Independence Day as would be expected for that time of year.
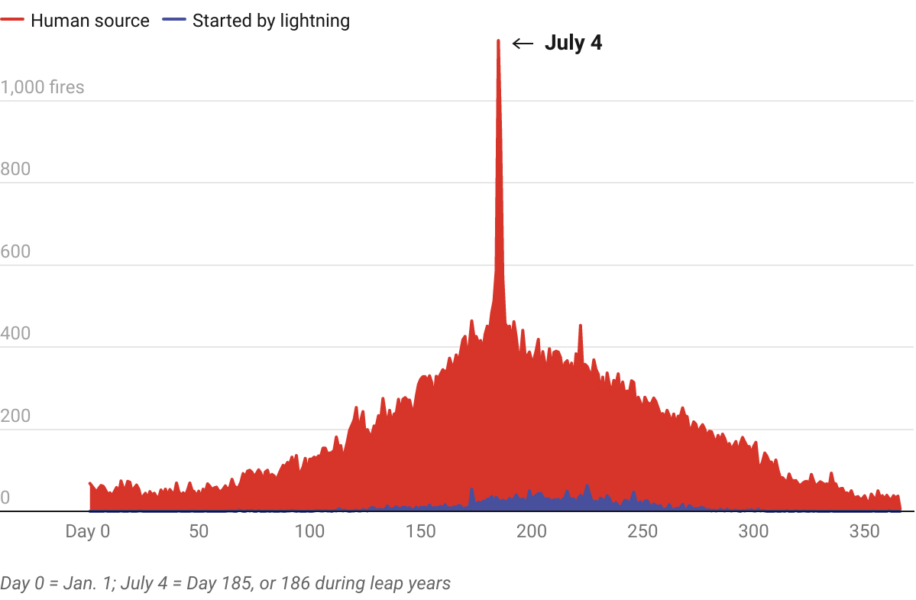
Image: The Conversation, CC-BY-ND From Ref. [8]
In recent years, some cities have switched to using drones instead of fireworks for their celebrations. Hundreds of drones carrying LEDs can create far more intricate designs in the sky than would be possible with fireworks. The drones don’t pose a fire risk and are much quieter, so they are less likely to upset people, wildlife, and pets living nearby.

Image: Ars Electronica/Florian Voggeneder, CC BY-NC-ND 4.0
However, the whoosh, bang, and sparkle of a firework display is all part of the show. Is it worth it or is it time our love of fireworks fizzled out?
References
[1] Sources of potassium emissions in 2019 on the National Atmospheric Emissions Inventory: https://naei.beis.gov.uk/overview/pollutants?pollutant_id=116
[2] Moreno T et al. (2010) Effect of fireworks events on urban background trace metal aerosol concentrations: Is the cocktail worth the show? Journal of Hazardous Materials 183:945-949. doi:10.1016/j.jhazmat.2010.07.082
[3] Godri JK et al (2010) Particulate Oxidative Burden Associated with Firework Activity. Environ. Sci. Technol. 44:8295–8301 doi:10.1021/es1016284
[4] Moreno T et al (2007) Recreational atmospheric pollution episodes: inhalable metalliferous particles from firework displays Atmospheric Environment 5:913–22. doi:10.1016/j.atmosenv.2006.09.019
[5] European city air quality viewer https://www.eea.europa.eu/themes/air/urban-air-quality/european-city-air-quality-viewer.
[6] Mendoza DL, Benney TM, Boll S (2021) Long-term analysis of the relationships between indoor and outdoor fine particulate pollution: A case study using research grade sensors Science of The Total Environment 776:145778. doi:10.1016/j.scitotenv.2021.145778
[7] Wilkin RT, Fine DN, Burnett NG (2007) Perchlorate Behavior in a Municipal Lake Following Fireworks Displays Environ. Sci. Technol. 41:3966–3971. doi:10.1021/es0700698
[8] Mietkiewicz et al. (2020) In the Line of Fire: Consequences of Human-Ignited Wildfires to Homes in the U.S. (1992–2015). Fire 3:50. doi:10.3390/fire3030050
Resources
- Checkout this fantastic infographic on firework colours by Compound Interest.
- Learn how the extraction of metals can also have negative environmental effects: Furze J, Harrison T (2021) Elements in danger! Science in School 54.
- Explore the light emitted by different substances: Ribeiro CI, Ahlgren O (2016) What are stars made of? Science in School 37:34–39.
- Investigate the effect of fireworks on air quality with your class: Shallcross D, Harrison T (2011) Smoke is in the air: how fireworks affect air quality. Science in School 21:47–51.
- Explore meteorological phenomena and how they are affected by air pollution with these fun classroom activities: Bultitude K (2009) Take the weather with you. Science in School 11:52–57.
- Read about satellite data for weather forecasting and air quality measurements: Rider H, Straume AG (2019) Forecasts from orbit. Science in School 46:14–19.
- Find classroom activities on how to use and control fire: Krzeczkowska M, Grygo-Szymanko E, Świt P (2016) Practical pyrotechnics. Science in School 38:46–51.
- Watch this video of flame colours from different metal salts.
- Explore the flame test safely in your classroom.

