Colourful chemistry: redox reactions with lollipops Teach article
Use a lollipop to activate colour-changing redox reactions in this simple but eye-catching activity.
Teaching oxidation-reduction (redox) reactions is part of all secondary-school chemistry curricula. In this article, we describe a vivid colour-changing demonstration to illustrate a chain of redox reactions, whereby electrons are transferred between different compounds and ions. The activity is suitable as a teacher demonstration, or older students could carry out the experiment themselves.

IanRedding/Shutterstock.com
Oxidising and reducing agents
A redox reaction is any chemical reaction in which a molecule, atom or ion loses or gains electrons, altering its oxidation state. An oxidising agent gains electrons (and is reduced in the reaction) and a reducing agent loses electrons (and is oxidised in the reaction). In this experiment, glucose from a lollipop is used as the reducing agent. When glucose is added to a solution containing OH– ions, a variety of half-reactions occur, one of which involves aldehyde groups in the glucose donating electrons. This gives rise to carboxylic groups in the form of carboxylate (due to the alkaline medium):
–CHO + 3 OH– → –COO– + 2 H2O + 2 e–
(aldehyde group) (carboxylic group, as carboxylate)
Moreover, alcohol groups in the glucose also donate electrons, giving rise to carboxylic groups (in the case of primary alcohols) and ketone groups (in the case of secondary alcohols):
–C(H)(OH)– + 2OH– → –C(=O)– + 2H2O + 2e–
(secondary alcohol) (ketone group)
In our experiment, glucose is added to a permanganate solution together with sodium hydroxide (NaOH), so electrons from glucose (C6H12O6) are first donated to permanganate ions (MnO4–). The oxidation products of the reducing sugar are mainly glucuronic acid (C6H10O7), plus some arabinonic acid (C5H10O6) and formic acid (CH2O2). If the lollipop is made from fructose, which is an isomer of glucose, the main product is fructonic acid (also C6H10O7).
In a series of redox reactions, electrons are donated continually from glucose to successive compounds of manganese. At each step in the chain, a colour change is visible. Manganese is ideal for this experiment, as it has more stable oxidation states than any other transition metal (from +2 to +7), each of which has a different colour.
You may be familiar with the classic ‘chemical chameleon’ demonstrationw1, of which this experiment is an adaptation. In the original version, you begin with a potassium permanganate and glucose solution, which changes colour upon mixing with a spatula. By using a lollipop instead, the glucose is added more gradually to the solution, which makes it easier to follow the colour changes. Using a miniature electric whisk means the lollipop is stirred faster than by hand.
Materials
You will need the following materials (see figure 1):
- Potassium permanganate (KMnO4) crystals
- Spherical lollipop containing glucose (or other reducing sugar, e.g. fructose)
- 3–4 sodium hydroxide (NaOH) pellets (approximately 0.5 g)
- 200 ml distilled water
- 250 ml conical flask or beaker (glass or plastic)
- Spoon and spatula
- Miniature electric whisk, e.g. hand-held milk frother
- Adhesive tape

Marisa Prolongo
Safety note
A lab coat, gloves and safety goggles should be worn. Teachers should follow their local health and safety rules, in particular concerning the use of potassium permanganate and the disposal of the resulting solution. See also the general safety note.
Procedure
The activity is suitable for a single lesson. The experiment takes only about 15 minutes and can be followed up by a set of discussion questions.
The steps are as follows:
- Fill the flask or beaker with 200 ml of distilled water.
- Stir in the NaOH pellets with the spoon until they have dissolved completely.
- Using the spatula, add a few potassium permanganate crystals (not too many, or the colour will be too dark to see the changes). When potassium permanganate (KMnO4) is added to the alkaline NaOH solution, it dissolves into potassium (K+) and permanganate (MnO4–) ions.
- Attach the stick of the unwrapped lollipop to the mini electric whisk using adhesive tape (see figure 1).
- Insert the lollipop into the solution and switch on the whisk to start mixing.
As the lollipop dissolves into the solution, you will observe colour changes for each redox reaction. The first two changes happen very rapidly (3–5 seconds), while further changes take a little longer. Students can take photos (e.g. with the camera of a mobile phone) at various time points to better compare and follow the changes in colour. A video from the authors demonstrating the experiment is available in Spanishw2.
What happens in the experiment?
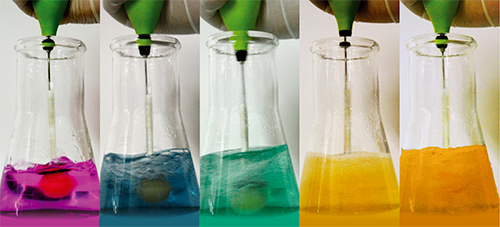
As the lollipop dissolves in the solution containing manganese ions, at least five different colours can be distinguished (as shown in figure 2), which correspond to different oxidation states of manganese.
- The first colour (purple) corresponds to permanganate ions (MnO4–). Manganese has the oxidation state +7.
- The permanganate ions (MnO4–) are then reduced to manganate ions (MnO42–). The oxidation state of manganese changes from +7 to +6, and the colour changes from purple to green. MnO4–(aq) (purple) + e– → MnO42–(aq) (green)An intermediate blue stage occurs between steps 1 and 2. One explanation is that the mixture contains both the purple permanganate (MnO4–) and the green manganate ions (MnO42–), which combine to produce a blue solution. Another explanation is that a part of permanganate is reduced to hypomanganate (MnO43–), which has an oxidation state of +5 and a blue colour.MnO4–(aq) (purple) + 2e– → MnO43–(aq) (blue)
- The manganate ions (MnO42–), which have an oxidation state of +6, are further reduced to manganese dioxide (MnO2), with an oxidation state of +4, causing a colour change from green to yellow-brown. MnO42–(aq) (green) + 2 H2O(l) + 2e– → MnO2(s) + 4OH–(aq) (yellow-brown)
- Finally, when even more glucose is incorporated into the solution, brown-black manganese dioxide (MnO2) forms a colloidal suspension in alkaline solution, which (if fairly dilute) can appear orange.
Marisa Prolongo
Variations in colour
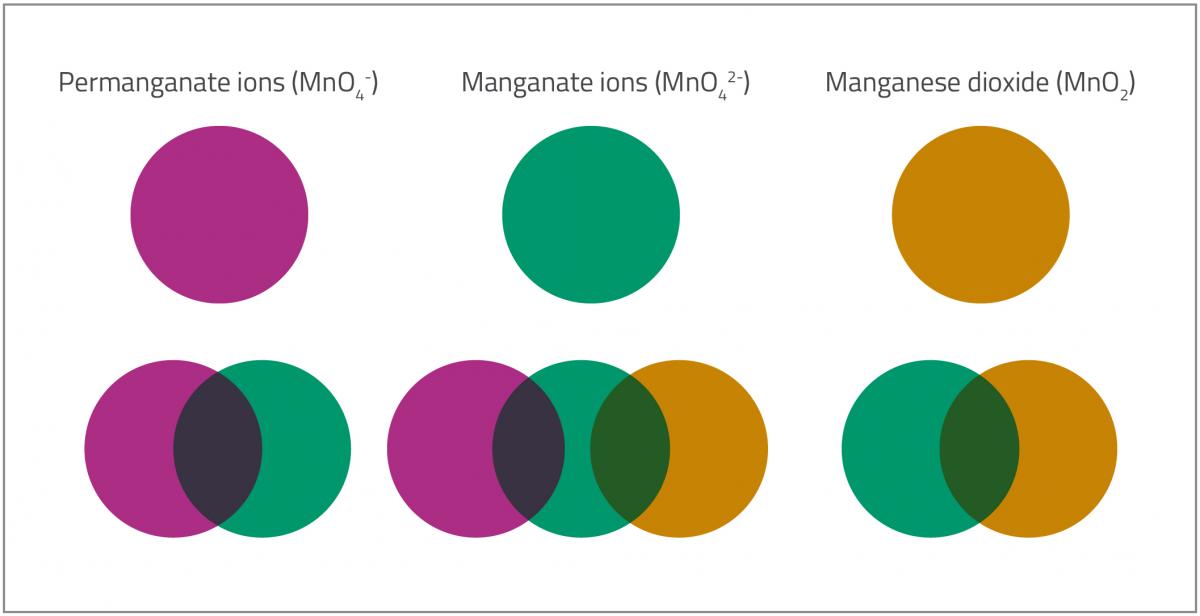
The food colours used in lollipops do not have a big impact on the colours you see in this experiment, but some other factors do play a role. Once the reactions start, there are always mixtures of ions in the solution, resulting in mixtures of colours that are not always easy to interpret (see figure 3).
Another factor is that the colour of manganese ions in solution is generally different from the colour of their corresponding solid salts. This is because manganese ions form complexes with water due to the electron-accepting capacity of their atomic d-orbitals. In addition, the tendency for molecules to accept electrons varies with pH and temperature, so if you change these variables or the quantities of the chemicals, the colours will vary, and the colour changes will occur at different times between experiments.
Nicola Graf
Electron configuration and transition metals
Electrons are arranged in energy levels called shells. Each shell is divided into subshells, which are made up of orbitals. Transition metals have one or more electrons in their outermost d-orbital. The difference in energy between individual d-orbital electrons is relatively small, so all transition metal cations have a variety of ways of forming chemical bonds involving different numbers of d-orbital electrons. This is why transition metals have several oxidation states.
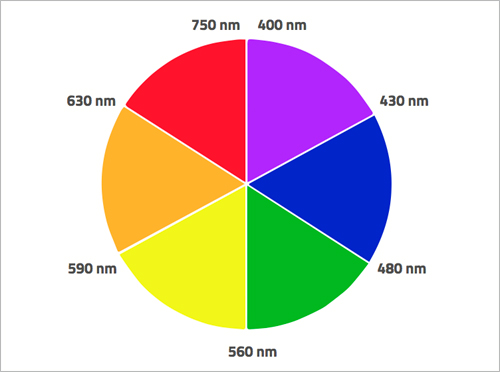
When electrons absorb certain frequencies of electromagnetic radiation, they jump to a higher energy level. In many transition metals, the difference in energy between d-orbitals corresponds to the energy of radiation of the visible light spectrum. For example, the d-orbital electrons of permanganate ions absorb electromagnetic radiation from the yellow part of the visible spectrum, but what we see as the colour of permanganate ions is the colour complementary to yellow – that is, purple. We see the colour of the remaining wavelengths that were not absorbed (figure 4).
Nicola Graf
Discussion
To link the lollipop demonstration to the chemistry of redox reactions, ask your students some of the following questions:
- In the experiment, what is the reducing agent that donates electrons in the redox reactions?
This depends on which reducing sugar you use, but in our experiment, the reducing agent is glucose (C6H12O6). - What is the oxidising agent that accepts the electrons?
The first oxidising agent in the reaction is the permanganate ions. After that, electrons are donated to manganate ions. - Does potassium manganate in solution absorb the part of visible light that corresponds to the colour we see (green) or to its complementary colour (red)?
Potassium manganate absorbs electromagnetic radiation from the red part of the visible spectrum, but what we see as the colour of manganate ions is the complementary colour, green. - Do you know of any other chemical elements that show different colours in different oxidation states in solution?
Examples include: chromium (Cr2O72–, orange; CrO42–, yellow) and vanadium (V2+, violet; V3+, green; VO2+, blue; VO43–, yellow) - What are the main uses of manganese, for example in biology or industry?
Manganese compounds are used in stainless steels and batteries, and as fuel additives and pigments. Manganese is also an essential cofactor for many enzymes, such as photosystem II in chloroplasts. However, in large amounts it is toxic to humans.
Variations of the experiment
This experiment can be performed in a number of different ways. For example, rather than using a lollipop, you could use a chewing gum containing sugar as the reducing agent; or instead of adding the glucose to a flask, you could add it to a plastic bottle and shake it to observe the colour changes (see figure 5). Your students could use their creativity to think of alternative experiments.

Marisa Prolongo
Acknowledgements
This article is based on a presentation at the Spanish Science on Stage festival (Ciencia en Acción) in 2014. This work was first carried out by students at IES Manuel Romero Secondary School in Málaga, Spain. We are grateful for the support of the Technical University of Madrid (Universidad Politécnica de Madrid) for the projects ‘Promotion of experimental learning of chemistry’ and ‘Chem-Innova’, and of the Spanish Royal Society of Chemistry (Real Sociedad Española de Química, RSEQ).
Web References
- w1 – For variations of the experiment, visit the Science Brothers website and the Hobby Chemistry website.
- w2 – A video of the experiment is available in Spanish on YouTube and the IES Manuel Romero Secondary School website.
Resources
- For ideas on introducing redox reactions using everyday examples, see:
- Voak H (2016) Redox resources. Science in School 36.
- To try a colour-change experiment involving pH-sensitive plant dyes, see:
- Shimamoto GG, Vitorino Rossi A (2005) An artistic introduction to anthocyanin inks. Science in School 31: 32–36.
Institutions
Science on StageReview
The redox chemistry of manganese is a fascinating aspect of transition metal chemistry. This simple practical exercise helps students to familiarise themselves with the variable oxidation states of manganese and their respective colours.
Observing the different colours will elicit discussion and will be a point of focus for understanding what is happening in the redox steps in the reaction.
Andrew Galea, chemistry lecturer, Giovanni Curmi Higher Secondary School, Malta
