Exploding chromosomes: how cancer begins Understand article
Brain tumours are one of the most common causes of death in children – and may begin when chromosomes are torn apart during cell division.
TP53 gene appears to cause
chromosomal ‘explosions’
linked to cancer
Image courtesy of EMBL /
P Riedinger
It’s a scene that has played out in many a household: the whole family on hands and knees, chasing coloured beads, as a distraught child stands wide-eyed, holding the remnants of a favourite necklace.
Once most of the beads have been collected, a kind adult threads them onto a new cord, and the crisis is over. Unless, of course, the child won’t be satisfied with anything less than an exact replica of the original necklace: finding all the beads – including the ones that rolled under the sofa or behind the cupboard – and threading them back in the right order can be a tricky business.

iStockphoto
At Heidelberg University Hospital, Germany, Andreas Kulozik encountered a family with a much more serious problem: a little girl and her brother had developed highly aggressive tumours. In initial genetic tests, Andreas found that the siblings had the same mutation in the gene TP53. They had this mutation in all their cells, not just the cancerous ones, which meant it was inherited from their parents, rather than acquired later by the cells that formed the tumour.
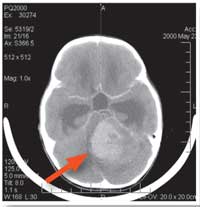
scan showing a
medulloblastoma (see arrow)
in the brain of a six-year-old
girl
Image courtesy of Reytan;
image source: Wikimedia
Commons
When Jan Korbel from the European Molecular Biology Laboratoryw1 teamed up with Stefan Pfister and Peter Lichter from the German Cancer Research Centerw2 to look at the genetics of childhood brain tumours, this family connection seemed a good place to start. As part of the International Cancer Genome Consortium, Jan, Stefan and Peter were sequencing the whole genome of cells from a childhood tumour for the first time. Called medulloblastoma, it is the most common of all malignant paediatric brain cancers, which are the most fatal cancers in children and the second most common cause of childhood deaths in developed countries after car accidents.
“When we got the DNA sequence data back, we saw a chaos in the girl’s genome that we couldn’t really explain at first,” says Tobias Rausch, from Jan’s research group, who led the data analysis. “Then we saw a paper by another group, describing a newly discovered phenomenon they called chromothripsis, and it clicked,” adds fellow group member Adrian Stütz.

image source: Flickr
The scientists realised they too were seeing chromothripsis, the cellular equivalent of the broken necklace scenario: a chromosome (sometimes two) had somehow exploded into countless small pieces, and had then been put back together with some pieces missing and others in the wrong order. As they analysed more samples, the scientists realised that this happened in the cancerous tissues of all medulloblastoma patients who carried any inherited TP53 mutation, but in none of the patients with normal TP53 or in the healthy tissue of the medulloblastoma patients.
“This makes us suspect that these three events are connected,” says Jan. “We believe that a TP53 mutation may cause chromosomes to explode, or possibly prevent the cell from reacting properly when they do. This somehow then leads to highly aggressive forms of cancer.”
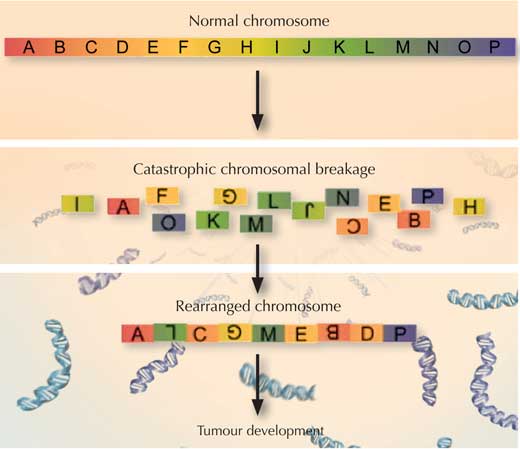
So how could a mutation in TP53 cause chromosomes to explode, and how would that lead to cancer? Scientists know that TP53 helps prevent chromosomes from fraying at the ends, by protecting telomeres – the caps that keep the ends of chromosomes together. If TP53 is faulty, Jan and colleagues speculate, telomeres could be compromised, and chromosomes could stick to each other.
In such a scenario, when that cell came to divide, chromosomes that were stuck together could run into problems. They would be pulled in opposite directions. At some point the strain would be too much, and, like the bead necklace that’s pulled too hard, one or both of the chromosomes would shatter, sending fragments of DNA flying. As the cell’s machinery raced to put the chromosome(s) back together, bits of genetic material might be left out and others re-assembled in the wrong order – or even from the wrong chromosome.

image source: Wikimedia
Commons
On the other hand, TP53 also plays a key role in inspecting our DNA for damage. If this guardian of the genome finds too many mistakes, it can push the cell into a programmed suicide (apoptosis) or into the cellular equivalent of old age (senescence), to prevent the cell from dividing and passing on those genetic defects.
But if TP53 is mutated, extensive damage to DNA could go unnoticed – damage such as a badly reassembled chromosome after chromothripsis, regardless of whether TP53 was involved in causing the chromosome explosion or not. As a result, oncogenes – genes that lead to cancer – could be activated, and the cell could start dividing and dividing, unchecked, thereby creating a tumour. Jan, Stefan and Peter speculate that these effects of a faulty TP53 may combine to lead to cancer in these patients, and would now like to investigate exactly how this is happening at each step.
In the meantime, their findings already have immediate repercussions for clinicians like Andreas and Stefan, and for their patients. “If a patient’s tumour cells show signs of chromothripsis, we now know that we should look for an inherited TP53 mutation,” Stefan says. And this is important, because having an inherited TP53 mutation could make the most commonly used cancer treatments backfire. Many chemo- and radiotherapy treatments kill cancer cells by damaging their DNA, but they also affect other cells in the body. In most patients, although this can lead to painful side effects, it does little long-term harm. Not so for someone with an inherited TP53 mutation. Because of that mutation, all of that person’s cells, including the healthy ones, will have trouble reacting to DNA damage.
So treatments that target DNA could actually make healthy cells turn cancerous, causing so-called secondary and tertiary tumours – “something we often see in patients with inherited TP53 mutations”, says Stefan. For such patients, it may be preferable to prescribe less intensive treatments using agents that do less damage to DNA. And, if a patient has an inherited TP53 mutation, this tells the doctor that that person’s immediate family should be tested, too. If any healthy family members carry the mutation, it should be seen as a signal for regular screening, as they are very likely to develop tumours at some point in their lives. “And the best chances of fighting cancer – especially the aggressive, early-onset types of cancer that seem to be associated with chromothripsis – are if it is diagnosed early,” Jan points out.
In fact, scientists think that 2-3 % of all cancers are probably caused by chromothripsis, so Jan’s group are now investigating whether TP53 mutations play a role in similar chromosome explosions in other tumours besides medulloblastoma. They have already found evidence for the same link between chromothripsis and inherited TP53 mutations in acute myeloid leukaemia. In this aggressive type of blood cancer in adults, Jan and colleagues discovered that patients with both a non-inherited TP53 mutation (i.e. a TP53 mutation only in their tumour cells) and evidence of chromothripsis tended to be elderly. The scientists point out that this makes sense in light of TP53’s role in telomere integrity. Our chromosome caps naturally get shorter as we age, making chromosome ends even more likely to get stuck to each other if TP53 goes awry. This in turn makes chromothripsis – and the ensuing cancer – more likely, the scientists suspect.
Jan’s group is continuing to explore these issues in brain, blood and other cancers, to unravel how faulty versions of TP53 are linked to chromosomes exploding like broken necklaces, as well as what other aspects of cells’ housekeeping efforts are involved in cancer.
More about EMBL

The European Molecular Biology Laboratory (EMBL)w1 is one of the world’s top research institutions, dedicated to basic research in the life sciences. EMBL is international, innovative and interdisciplinary. Its employees from 60 nations have backgrounds including biology, physics, chemistry and computer science, and collaborate on research that covers the full spectrum of molecular biology.
EMBL is a member of EIROforumw3, the publisher of Science in School.
References
- The research results were published in:
- Rausch T et al. (2012) Genome sequencing of pediatric medulloblastoma links catastrophic DNA rearrangements with TP53 mutations in cancer. Cell 148(1-2): 59-71
Web References
- w1 – Learn more about the European Molecular Biology Laboratory.
- w2 – The German Cancer Research Center (Deutsches Krebsforschungszentrum) is the largest biomedical research institute in Germany, where over 1000 scientists investigate the mechanisms of cancer, identify cancer risk factors and try to find strategies to prevent people from getting cancer.
- w3 – EIROforum is a collaboration between eight of Europe’s largest inter-governmental scientific research organisations, which combine their resources, facilities and expertise to support European science in reaching its full potential. As part of its education and outreach activities, EIROforum publishes Science in School.
Resources
- For a teaching activity about how geneticists identify cancerous cells, see:
- Communication and Public Engagement Team (2010) Can you spot a cancer mutation? Science in School 16: 39-44.
- To learn how cancer stem cells may revolutionise the treatment of cancer, see:
- Mazza M (2011) Cancer stem cells – hope for the future? Science in School 21: 18-22.
- For more information on how genetic mutations cause diseases, see:
- Patterson L (2009) Getting a grip on genetic diseases. Science in School 13: 53-58.
- For a classroom activity about knowing what your genes have in store for you, including the possibility of cancer, see:
- Strieth L et al. (2008) Meet the Gene Machine: stimulating bioethical discussions at school. Science in School 9: 34-38.
Institutions
Review
The article is written in a clear and concise manner with an interesting hook to set the scenario. The analogy of exploding chromosomes and broken necklaces is very apt; it can help students understand the difficult job of putting an exploded chromosome back together again in its original form. It is feasible to carry out a demonstration of this at school, since beads are fairly standard pieces found in a school biology laboratory.
This article can be used as an extension activity to the teaching of genetic mutations or to extend discussion of the role of genes in cancer. It can also be used as a basis for further research into TP53 mutations, or the role of genes in cancer and childhood medulloblastomas for students who are interested in becoming medics. Used in conjunction with appropriate questions, this article can be used as a comprehension exercise with plenty of opportunity for further web-based investigations into mutations, oncogenes, types of cancers, cancer treatments and telomeres.
Suitable comprehension questions include:
- If a mutation is found in all cells, is it more likely to be a random mutation or one inherited from parents? Explain your answer.
- What is a medulloblastoma? Explain why it is so malignant.
- Draw a series of diagrams to show what chromothripsis is.
- What are two roles of the TP53 gene?
- What are two possible mechanisms by which a TP53 mutation causes cancer?
- What are oncogenes?
- How does the TP53 mutation affect cancer treatments?
- What is the role of telomeres in chromosomes?
Students could also use the article to construct a glossary of uncommon words and concepts.
Shaista Shirazi, UK
